STIMUFEND® (pegfilgrastim-fpgk): Established biosimilarity to Neulasta® (pegfilgrastim)
Proven structural, functional, and clinical similarity1,2
- STIMUFEND is FDA approved based on the totality of evidence supporting its biosimilarity to Neulasta
Confirmed bioequivalence to Neulasta1
PK parameters confirmed bioequivalence from post-dose
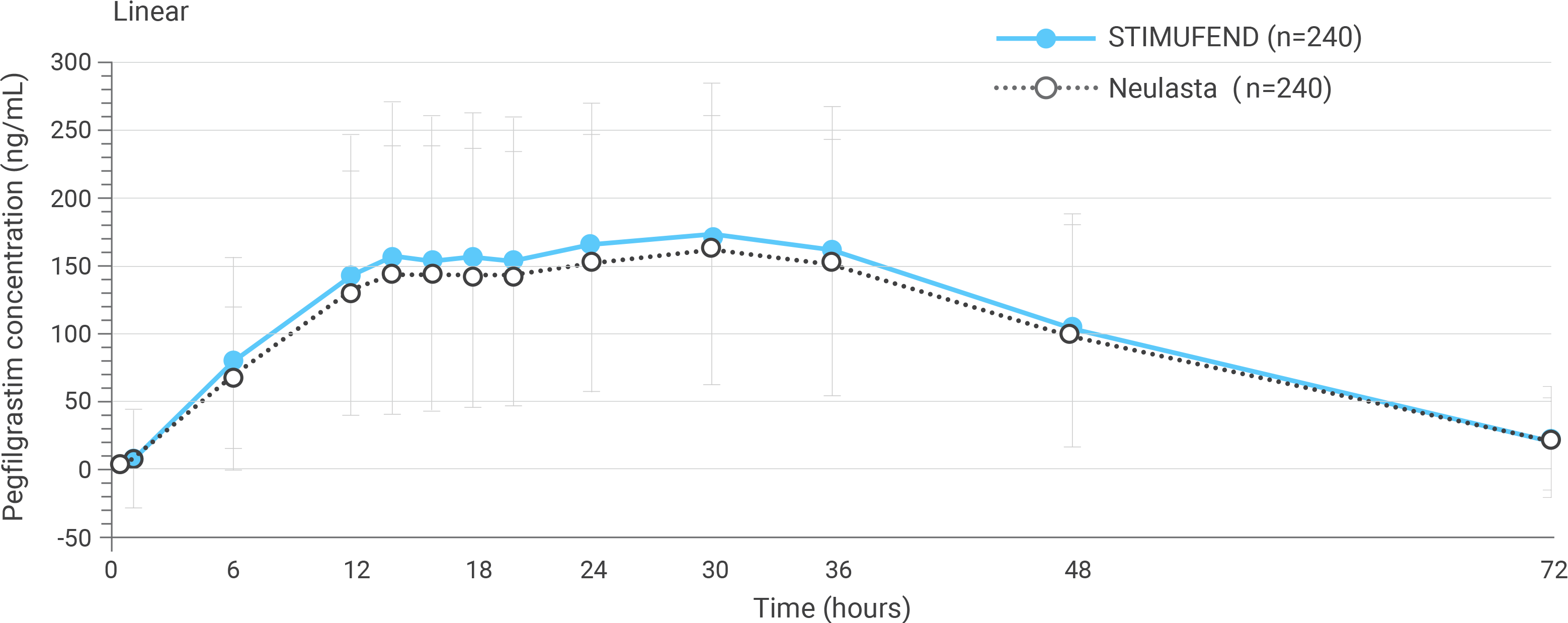
Arithmetic mean (SD) pegfilgrastim serum concentration time profiles after a single dose of pegfilgrastim in healthy subjects.
PD parameters confirmed bioequivalence over 16 days from post-dose
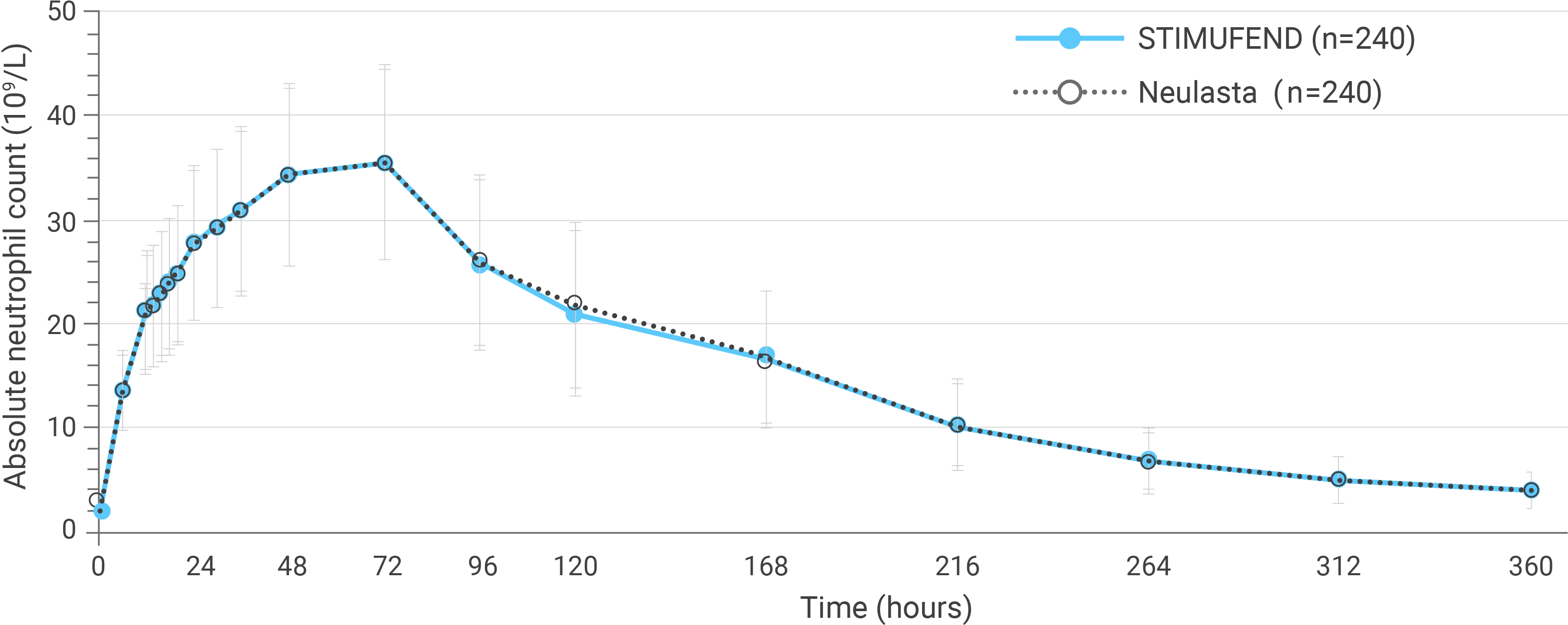
Arithmetic mean (SD) observed ANC-time profiles after a single dose of pegfilgrastim in healthy subjects.
- STIMUFEND was assessed in healthy subjects in a double-blind, randomized, 2-sequence, 2-period, 2-treatment, crossover Phase I study (NCT03251248)1
- 240 subjects received both treatments and were included in the PK/PD analysis sets
- Primary PK endpoints were AUC from time zero to the last sampling time, AUC from time zero extrapolated to infinity, and Cmax
- Primary PD endpoints were Emax and AUE from time zero (pre-dose) to time to last quantifiable concentration for ANC
- For all primary PK/PD parameters, the 90% repeated confidence intervals of the geometric means ratio of STIMUFEND to Neulasta were entirely within the equivalence range (80%-125%), confirming bioequivalence1
Inclusion Criteria
- Heathy males and nonpregnant females
- Aged ≥18 to ≤55 years
- Body mass index ≥18.0 to ≤29.9 kg/m2
- In good health*
- Provide signed informed consent
Exclusion Criteria
- Signs or symptoms of COPD
- Smoking > 10 cigarettes/day
- Prior exposure to any CSF or growth factor 3 months (84 days) before randomization
- Prior exposure to therapeutic mAbs targeting the bone marrow or blood cells
- Exposure to mAbs not affecting bone marrow or blood cells was allowed before screening†
- Blood (≥500 mL) or plasma donation within 3 months (84 days)
- Stem cell or bone marrow donation within 12 months (336 days) before screening
Patients who were positive for anti-PEG antibodies at screening could be included in the study.1
*Based on comprehensive medical history, physical examination, vital signs, and clinical laboratory tests.
†If mAbs discontinued > 3 months (84 days) or 5 half-lives (whichever was longer).
View the clinical trial article by Lickliter, et al in Clin Ther. ![]()
Similar immunogenicity to Neulasta2
- STIMUFEND demonstrated non-inferiority to Neulasta with confirmed immunogenicity rates of 8.9% and 9.5% respectively
- The immunogenicity and safety profiles of STIMUFEND were demonstrated in healthy subjects in a double-blind, randomized, parallel-group, Phase I study (NCT03251339)2
- A total of 336 subjects were randomized and treated
- Primary endpoints were confirmed treatment-induced ADA-positive status and confirmed NAb status to pegfilgrastim from predose until the end of study
- STIMUFEND demonstrated non-inferiority with Neulasta with confirmed immunogenicity rates of 8.9% and 9.5%, respectively2
- No filgrastim-specific neutralizing antibodies were detected in either treatment group2
Immunogenicity with ADA rates at any time after the first dose of 2 single doses2
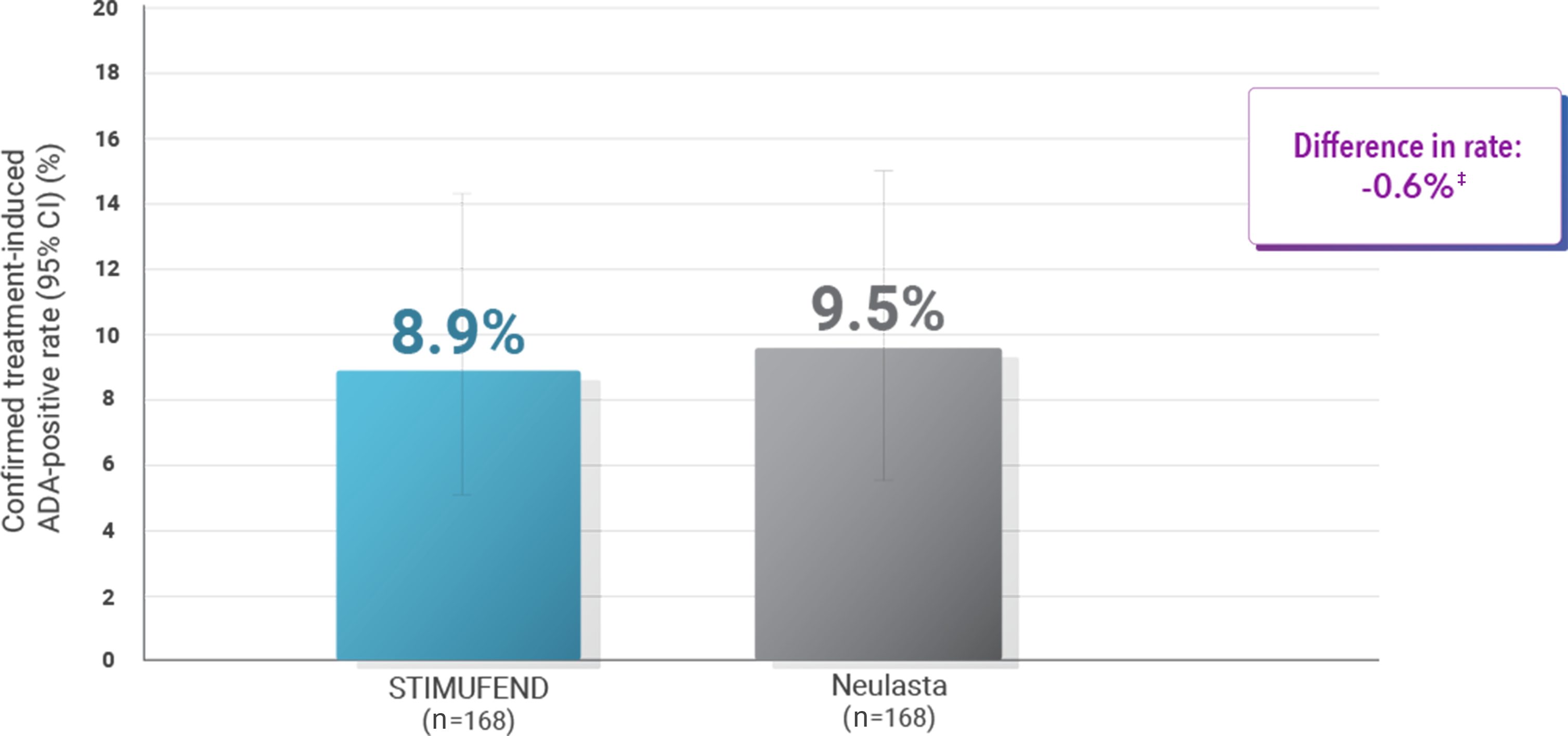
‡Upper limit of the exact one-sided adjusted 95% CI: 6.25. Confidence interval (CI) determined using Clopper-Pearson method.
Inclusion criteria2
- Heathy males and nonpregnant females
- Aged ≥18 to ≤55 years
- Body mass index ≥18.0 to ≤29.9 kg/m2
- In good health*
- No known hypersensitivity to any component of either pegfilgrastim product
- All subjects were required to comply with the contraception requirements specified in the clinical study protocol
- Provide signed and dated written informed consent
Exclusion criteria2
- Signs or symptoms of COPD
- Smoking > 10 cigarettes/day
- Splenomegaly (spleen size > 13 cm in the craniocaudal dimension by US)
- Prior exposure to any CSF or growth factor 3 months (84 days) before randomization
- Prior exposure to therapeutic mAbs targeting the bone marrow or blood cells
- Exposure to mAbs not affecting bone marrow or blood cells allowed before screening†
- Blood (≥500 mL) or plasma donation within 3 months (84 days)
- Stem cell or bone marrow donation within 12 months (336 days) before screening
*Based on comprehensive medical history, physical examination, vital signs, and clinical laboratory tests.
†If mAbs discontinued > 3 months (84 days) or 5 half-lives (whichever was longer).
Highly similar safety and tolerability profile
Most common TEAEs (>5% of subjects)
| TEAEs | STIMUFEND (n=270) n (%) |
Neulasta (n=266) n (%) |
|---|---|---|
| Headache | 151 (55.9) | 150 (56.4) |
| Musculo-skeletal pain | 133 (49.3) | 114 (42.9) |
| Bone pain | 67 (24.8) | 70 (26.3) |
| Back pain | 45 (16.7) | 55 (20.7) |
| Upper respiratory tract infection | 32 (11.9) | 20 (7.5) |
| Nausea | 30 (11.1) | 31 (11.7) |
| Injection-site pain | 28 (10.4) | 25 (9.4) |
| Myalgia | 27 (10.0) | 23 (8.6) |
| Neutro-penia | 24 (8.9) | 22 (8.3) |
| Abdominal pain | 23 (8.5) | 21 (7.9) |
| Palpita-tions | 23 (8.5) | 14 (5.3) |
| Injection-site bruising | 17 (6.3) | 18 (6.8) |
| Abdominal pain upper | 13 (4.8) | 19 (7.1) |
| Leuko-cytosis | 13 (4.8) | 14 (5.3) |
- Adverse events with STIMUFEND were consistent with the administration of Neulasta1
- TEAEs were generally mild to moderate in severity and were self-limiting1
- Clinically significant splenomegaly* (Grade 1 or 2): STIMUFEND (n=3); all resolved spontaneously with no further study drug administered1
*Rare cases of splenic rupture have been reported following administration of filgrastim or pegfilgrastim; therefore, the spleen was monitored throughout the study.
Most common TEAEs (>5% of subjects)
| TEAEs | STIMUFEND (n=168) n (%) |
Neulasta (n=168) n (%) |
|---|---|---|
| Headache | 105 (62.5) | 120 (71.4) |
| Bone pain | 113 (67.3) | 101 (60.1) |
| Spinal pain | 67 (39.9) | 68 (40.5) |
| Upper respiratory tract infection | 32 (19.0) | 20 (11.9) |
| Nausea | 32 (19.0) | 19 (11.3) |
| White blood cell count increased* | 23 (13.7) | 27 (16.1) |
| Myalgia | 19 (11.3) | 17 (10.1) |
| Vomiting | 18 (10.7) | 9 (5.4) |
| Musculo-skeletal chest pain | 12 (7.1) | 17 (10.1) |
| Abdominal pain | 9 (5.4) | 15 (8.9) |
| Diarrhea | 8 (4.8) | 15 (8.9) |
| Oropharyngeal pain | 12 (7.1) | 14 (8.3) |
| Injection-site bruising | 12 (7.1) | 10 (6.0) |
| Arthralgia | 11 (6.5) | 11 (6.5) |
| Dizziness | 11 (6.5) | 11 (6.5) |
| Contusion | 11 (6.5) | 7 (4.2) |
| Fatigue | 7 (4.2) | 11 (6.5) |
| Back pain | 8 (4.8) | 9 (5.4) |
- TEAEs were generally mild to moderate in severity, self-limiting, and resolved without sequelae2
- Both instances of splenomegaly (Grade 1 and 2, respectively) resolved spontaneously, and the patients completed the study2
*All events considered related to study drug.
ADA, antidrug antibody; ANC, absolute neutrophil count; AUC, area under the curve; AUE, area under the effect-time curve; Cmax, maximum concentration; COPD, chronic obstructive pulmonary disease; CSF, colony-stimulating factor; Emax, maximum observed effect; FDA, US Food and Drug Administration; mAb, monoclonal antibody; NAb, neutralizing antibody; PD, pharmacodynamic; PEG, polyethylene glycol; PK pharmacokinetic; SD, standard deviation; TEAE, treatment-emergent adverse event.
References:
- Lickliter J, Kanceva R, Vincent E, et al. Pharmacokinetics and pharmacodynamics of a proposed pegfilgrastim biosimilar MSB11455 versus the reference pegfilgrastim Neulasta in healthy subjects: a randomized, double-blind trial. Clin Ther. 2020;42(8):1508-1518.e1. doi:10.1016/j.clinthera.2020.05.020
- Wynne C, Schwabe C, Vincent E, et al. Immunogenicity and safety of a proposed pegfilgrastim biosimilar MSB11455 versus the reference pegfilgrastim Neulasta® in healthy subjects: a randomized, double-blind trial. Pharmacol Res Perspect. 2020;8(2):e00578. doi:10.1002/prp2.578

